Testing times…
In the first of a mini-series on field tests and laboratory analysis, we consider the various techniques available to aid the investigation of coatings failures.
One of the principle techniques for checking and assessing coatings includes tests for water soluble salts. Surface-based residual water-soluble salts can impact on the performance of a protective coating. The higher the concentration of salt, which can accelerate the corrosion process, the bigger the risk of the coating failing. For most new construction and maintenance projects, water soluble salt testing based around extraction and analysis, is undertaken and can involve:
Bresle test (conductivity)
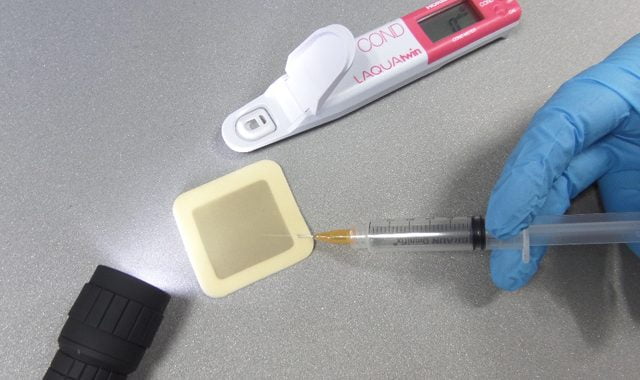
Consisting of a self-adhesive plastic patch with a central membrane of known surface area, the Bresle test is among the most common methods used to extract water soluble salt contaminants from a surface for analysis. The patch is placed onto the substrate, creating a seal. Then three millilitres of distilled or deionised water is injected through the foam edge using a needle and syringe. The water is injected, removed and re-injected at least four times in line with ISO8502-6 and then checked for conductivity using a meter, which provides a reading in μS/cm or ms/m.
Chloride ion test
This test is used to check for chloride ions and is based on an ion detection tube method. This involves placing a volume of patented extraction solution into a latex sleeve, which is fixed onto the surface under test. It is then massaged for two minutes to ensure that the solution is in contact with the substrate.
An ion detection tube is placed into the solution in the container and when the solution reaches the top of the tube, it is removed from the liquid. The chloride concentration is shown by a change in colour of the reagent in the tube and the value is shown on the scale on the side of the tube. The values are provided in ppm (parts per million) and converted into μg/cm² or mg/m² using the factor: 1 μg/cm² = 1 ppm = 10 mg/m².
Salt contamination meter
The conductivity filter paper method of salt detection can be used, which involves using filter paper soaked with 1.6ml of high purity water via a syringe and placed on the side of a magnetic disc. The water from the syringe is then spread evenly across the surface. The magnetic disc with wetted filter paper is placed in position on the surface for two minutes and then removed and placed in the measuring instrument where the conductivity is displayed.
It’s also important to consider SSPC Guide 15 and ISO 8502 standards in tests for water soluble salts. For further information on field tests, refer Fitz’s Atlas of Coating Surveys at https://fitzsatlas.com/